Looking for a reliable way to streamline operations and reduce costs? This comprehensive guide to Business Process Outsourcing explores how outsourcing can help businesses improve efficiency, reduce overhead, and focus on core goals.
Outsourcing originated as a cost-saving strategy that allowed companies to enhance efficiency and scale operations by delegating certain processes to external providers. Business process outsourcing (BPO) companies help organizations streamline operations, reduce costs, and improve efficiency by handling non-core functions such as customer service, data entry, and accounting.
Basically, by outsourcing, organizations can pay full attention to other, core areas of their business. Business process outsourcing for small businesses allows them to access expert services, reduce operational costs, and focus more on core activities like growth and customer engagement. Advanced BPO solutions offer access to automation, AI-driven solutions, and data analytics, enabling client companies to gain competitive advantage.
This post explores business process outsourcing benefits, services, and how to choose the right outsourcing partner.
Key Benefits of BPO
- Cost Savings
Outsourcing business process management helps businesses achieve cost reduction in operations while improving efficiency. By outsourcing non-core activities to third-party service providers, businesses can focus on their core competencies and reduce their overheads such as office equipment, rented workspace, recruitment and training, etc. Offshore outsourcing provides access skilled labor at significantly lower rates.
- Access to Specialized Expertise
BPO providers often bring industry-specific knowledge and best practices to the table for a wide range of services– from data entry and document conversion to ebook conversion and legal document processing — across all industries. For example, a legal firm that needs to convert thousands of case files from scanned PDFs into editable and searchable Word documents can outsource the task to a BPO provider with expertise in legal document conversion. Using OCR (Optical Character Recognition) technology customized for legal terminology and formatting, the provider can ensure high accuracy and preservation of legal citations, footnotes, and section numbering. Thanks to the BPO’s industry-specific expertise, the legal firm receives highly accurate, court-ready documents quickly and cost-effectively.
- Technology Integration
Technological advancements have significantly accelerated the growth of the BPO industry, enabling the adoption of automation, data analytics, and AI-powered solutions that drive greater efficiency and elevate the customer experience. From robotic process automation (RPA) to predictive analytics, BPO providers leverage advanced technologies to streamline operations and deliver tangible, data-driven business results.
- Improved Process Efficiency
The primary motivation for partnering with a BPO provider is to enhance efficiency by outsourcing time-consuming and resource-intensive processes. Tasks such as technical support, customer service, and other back-office functions are commonly delegated to BPO companies for this reason. Offshoring these operations offers additional advantages—such as cost savings through favorable exchange rates and the ability to leverage different time zones to maintain 24/7 productivity.
- Enhanced Customer Service
BPO can greatly enhance customer service by giving businesses access to skilled professionals who specialize in various support functions, including live chat, technical assistance, and multichannel communication. By outsourcing these services to a dedicated BPO provider, companies can ensure consistent, high-quality customer interactions—resulting in improved satisfaction, stronger brand loyalty, and better overall customer retention.
- Strategic Agility and Business Scalability
In today’s fast-paced digital landscape, agility is essential for staying competitive and seizing new opportunities. It reflects how quickly a business can adapt to market changes and evolving customer demands. By leveraging external service providers, companies can scale operations more efficiently, respond faster to shifts in the market, and maintain a competitive edge. With competitors just a click away, the agility to capture and retain customer attention has become more critical than ever.
- Enhanced Focus on Core Competencies
By outsourcing time-intensive functions like customer support and back-office operations to BPO providers, companies can redirect their internal resources toward core business objectives—driving innovation, enhancing productivity, and strengthening their competitive edge.
- Risk Management and Regulatory Compliance
Nearshore BPO services help businesses manage risk and maintain regulatory compliance more effectively. Outsourcing providers bring deep knowledge of industry-specific regulations and standards, ensuring that processes are carried out in full compliance with legal requirements. This specialized expertise significantly reduces the risk of non-compliance and associated legal complications. Moreover, BPO firms typically implement advanced security protocols to safeguard sensitive data, helping to minimize the risk of data breaches, cyber threats, and other security vulnerabilities.
Comprehensive BPO Services to Streamline Business Operations
BPO solutions are available for a wide range of tasks, allowing organizations to leverage expertise and reduce costs while focusing on their core operations.
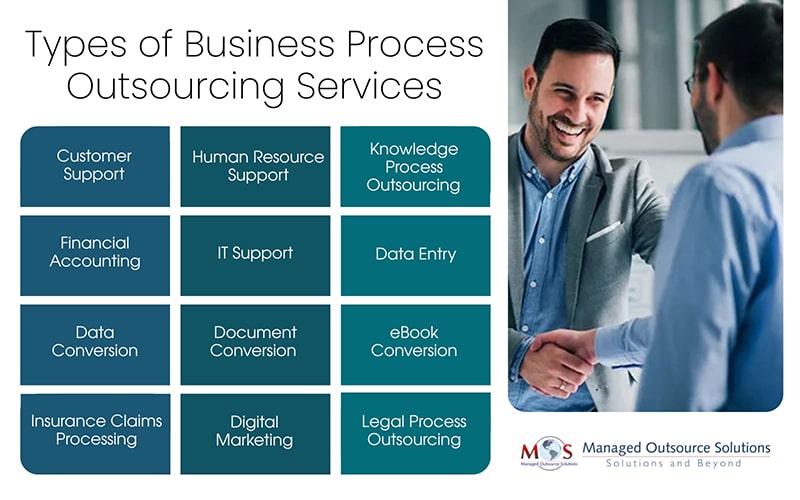
Types of business process outsourcing services explained:
- Customer Support
Customer support involves assisting customers by addressing inquiries, resolving issues, and providing help through channels like phone, email, live chat, and social media. BPO customer support teams are focused on delivering positive experiences and maintaining high levels of client satisfaction.
- Human Resource Support
HR outsourcing covers key functions such as employee management, payroll processing, recruitment, and administrative support. By outsourcing HR tasks, companies can streamline workforce management and stay compliant, while concentrating on strategic business goals.
- Knowledge Process Outsourcing (KPO)
KPO refers to the outsourcing of knowledge-intensive tasks that require domain expertise, such as research, data analysis, and intellectual property services. KPO providers add value by leveraging specialized knowledge and delivering insights that support critical decision-making.
- Financial Accounting
Financial accounting services include bookkeeping, transaction management, and ensuring compliance with accounting standards. Outsourcing these functions helps organizations maintain accurate financial records and improve the efficiency of financial operations.
- IT Support
IT support services cover technical assistance, software and hardware maintenance, network administration, and help desk services. Outsourcing IT support gives businesses access to expert resources, enhanced system uptime, and cost savings.
- Data Entry
Data entry outsourcing to experts ensures the accurate input of various types of information into digital systems, such as customer data, sales records, and inventory logs. Outsourcing data entry improves operational efficiency and allows businesses to focus on higher-value activities.
- Data Conversion
Data conversion entails transforming data from multiple formats or sources into a unified, structured digital format. This may include digitizing paper documents or migrating data across platforms. Outsourcing this task ensures consistency, accuracy, and better data management.
- Document Conversion
This involves converting files from one format to another to enhance accessibility, compatibility, and usability. Whether it’s converting paper documents into digital formats or transforming files between formats like PDF, Word, Excel, HTML, or XML, document conversion plays a critical role in streamlining data management and workflow efficiency. Businesses across industries rely on document conversion services to preserve data integrity, reduce manual entry, and ensure that information is easily searchable and shareable. With the rise of digital transformation, accurate and secure document conversion has become essential for organizations to stay agile and compliant.
- eBook Conversion
This is a specialized BPO service that involves transforming content from various formats—such as Word documents, PDFs, or scanned manuscripts—into widely accepted digital eBook formats like ePub, MOBI, AZW, or HTML5. As publishing moves increasingly toward digital platforms, outsourcing eBook conversion to experts in the field allows publishers, authors, and educational institutions to scale efficiently while ensuring professional formatting, device compatibility, and accessibility standards.
- Insurance Claims Processing
Insurance claims processing services handle tasks such as claim intake, data verification, adjudication, and policyholder communication. BPO providers streamline the claims lifecycle, improving turnaround times and reducing administrative burdens.
- Digital Marketing
Digital marketing BPO services include managing online channels like social media, content creation, email marketing, and SEO. Outsourcing these functions helps businesses boost their digital presence and effectively engage with target audiences.
- Legal Process Outsourcing (LPO)
LPO involves delegating legal tasks such as document review, legal research, and contract drafting to external experts. Outsourcing legal services helps law firms and corporate legal departments reduce costs and gain access to specialized legal expertise.
To benefit from the outsourcing explosion, businesses need to choose the right BPO partner.
How to Choose the Right BPO Partner for Your Company
Choosing the right outsourcing partner is a crucial decision that can greatly impact your business success. The right partner will not only deliver quality work but also align with your company’s values, goals, and expectations. Here are some key tips to help you make the best choice:
- Define Your Needs Clearly
Start by identifying what tasks or processes you want to outsource. Is it customer service, IT support, data entry, or something else? Knowing your exact needs helps you find a partner with the right skills and experience.
- Look for Industry Experience
Choose a provider that understands your industry. Experience with similar businesses means they’re more likely to anticipate your needs, follow regulations, and deliver results that make sense for your niche.
- Evaluate Technical Capabilities
Make sure the partner uses modern technology and tools. Whether it’s data security, automation, or real-time reporting, strong tech capabilities ensure smoother operations and better outcomes.
Choose a provider that embraces automation and AI. Modern outsourcing isn’t just about saving costs—it’s about working smarter. Look for a provider that leverages automation tools and AI-driven solutions to improve efficiency, reduce errors, and speed up routine tasks. From intelligent chatbots in customer service to automated data processing and predictive analytics, these technologies can give your business a competitive edge while ensuring consistent, high-quality outcomes.
- Check References and Reviews
Don’t hesitate to ask for client references or check online reviews, including case studies on successful BPO implementations. Feedback from other businesses gives you a clearer picture of what it’s like to work with the provider.
- Assess Communication and Support
Good communication is key. Choose a partner who responds promptly, understands your language and culture, and offers clear channels for ongoing support and collaboration.
- Consider Scalability and Flexibility
Your outsourcing needs may grow or change. Look for a provider who can scale with your business and adapt quickly when needed.
- Understand the Cost Structure
Conduct a cost comparison of in-house vs outsourced business functions before making the outsourcing decision. Then compare pricing models and understand what’s included. The cheapest option isn’t always the best—look for a balance between cost and quality.
- Check for Data Security and Compliance
Ensure the provider follows strict security protocols and complies with relevant regulations (like GDPR or HIPAA, if applicable). This is especially important if sensitive customer or business data is involved.
- Evaluate the Outsourcing Service Level Agreement (SLA)
Make sure the SLA sets clear expectations for both parties. A well-defined SLA helps ensure consistent service quality and reduces risk of disputes by defining recourse for underperformance. It provides legal protection and accountability.
- Know the Red Flags in BPO Partnerships
When assessing a BPO partner, be wary of these red flags:
- Lack of transparency in pricing, hidden fees, or unclear contract terms
- Poor communication: delays in responses, inconsistent updates or unclear points
- No defined SLA
- Unrealistic time lines or cost estimates
- Lack of continuity in service
- Poor tech infrastructure
- Negative client reviews
- No compliance or data sec certifications
- No proactive suggestions for improvement or efficiency.
BPO Growth – An Inevitable Phenomenon
According to a recent report, the global BPO market was valued at USD 314.4 billion in 2023 and is projected to grow from USD 332.01 billion in 2024 to USD 513.4 billion by 2032, reflecting a compound annual growth rate (CAGR) of 5.6% during the forecast period (2025–2032).
In 2025, business process outsourcing trends continue to evolve with the integration of AI and automation and the growth of virtual workplaces–redefining how businesses manage and elevate the customer experience. Organizations that place a strong emphasis on customer satisfaction consistently reap the rewards of increased loyalty and growth. Today’s BPO providers represent a new generation of innovative partners, equipped to deliver exceptional service through advanced cloud technologies, real-time analytics, personalized solutions, and secure operational models. Looking ahead, the expansion of the BPO industry is inevitable. By choosing the right partner, companies can stay competitive, agile, and efficient in a rapidly changing marketplace.